ENERGY FUTURE NEWS
Single-atom-thick sheets efficiently extract electricity from salt water
An impressive energy density generated by differences in salt concentrations.
It's possible to generate energy using nothing but the difference between fresh and salt water. When fresh and salt water are separated by a membrane that blocks the passage of certain ions, there is a force that drives the freshwater into the salt water to even out the salt concentration. That force can be harvested to produce energy, an approach termed "osmotic power."
But the generation of osmotic power is highly dependent on how quickly ions can cross the membrane—the thicker (and more robust) the membrane, the slower the ions will flow. Theoretically, the most efficient osmotic power generation would come from an atomically thin membrane layer. But can this theoretical system be achieved here in reality?
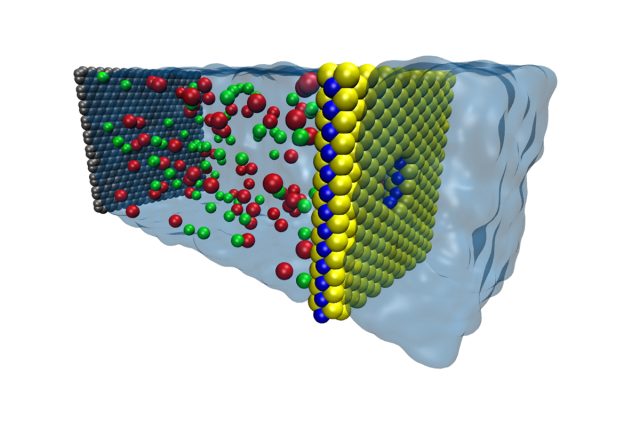
Recently, scientists answered that question using atomically thin membranes composed of molybdenum-disulfide (MoS2). In the paper that resulted, they describe a two-dimensional MoS2membrane containing a single nanopore, which was used to separate reservoirs containing two solutions with different concentrations of salt in order to generate osmotic power.
Understanding osmotic current
Not all ions can be transported through the nanopores of MoS2 membranes. Surface charges present around the pore limit ion diffusion, resulting in a selective ion transport that causes a measurable net osmotic current. In fact, the size of the osmotic current is actually determined by the surface charges present at the nanopore.
Analysis of experimental data at pH 5 revealed a negative surface charge at the site of the nanopore. As the pore size increases, more negative charges accumulate at the surface. This should repel negatively charged ions from the pore while allowing positively charged ions to cross. The result is a net positive current across the membrane.
The researchers also found that the conductance of the nanopore increases with increasing pH. They think this could be due to an increase in accumulation of negative surface charges in the nanopores. Similarly, increasing the pH increases the generated voltage and current, underlining the importance of the nanopore surface charge to ion movements.
